María de los Ángeles García-Robles
 Body weight control has become a critical public health issue
Body weight control has become a critical public health issue
Hence, a thorough understanding of how food intake is regulated could allow us to propose new strategies to stop the growing incidence of obesity in the global population. Since the arcuate nucleus is a master regulator of feeding, several groups have targeted this brain region to understand appetite control mechanisms better. Mainly, tanycytes have emerged as crucial hypothalamic cells to detect nutritional cues changes and respond by generating a compensatory response to regain homeostasis.
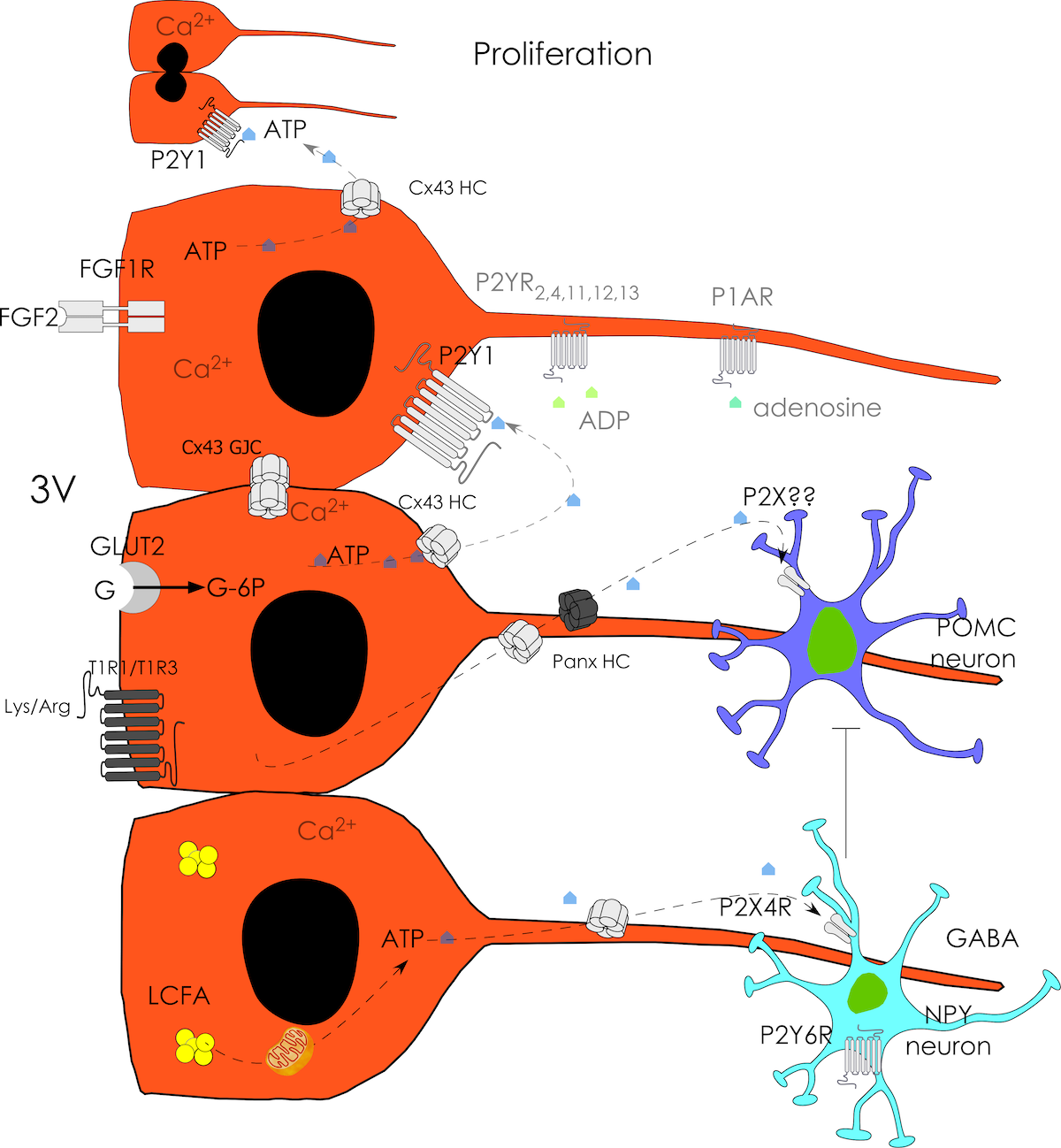
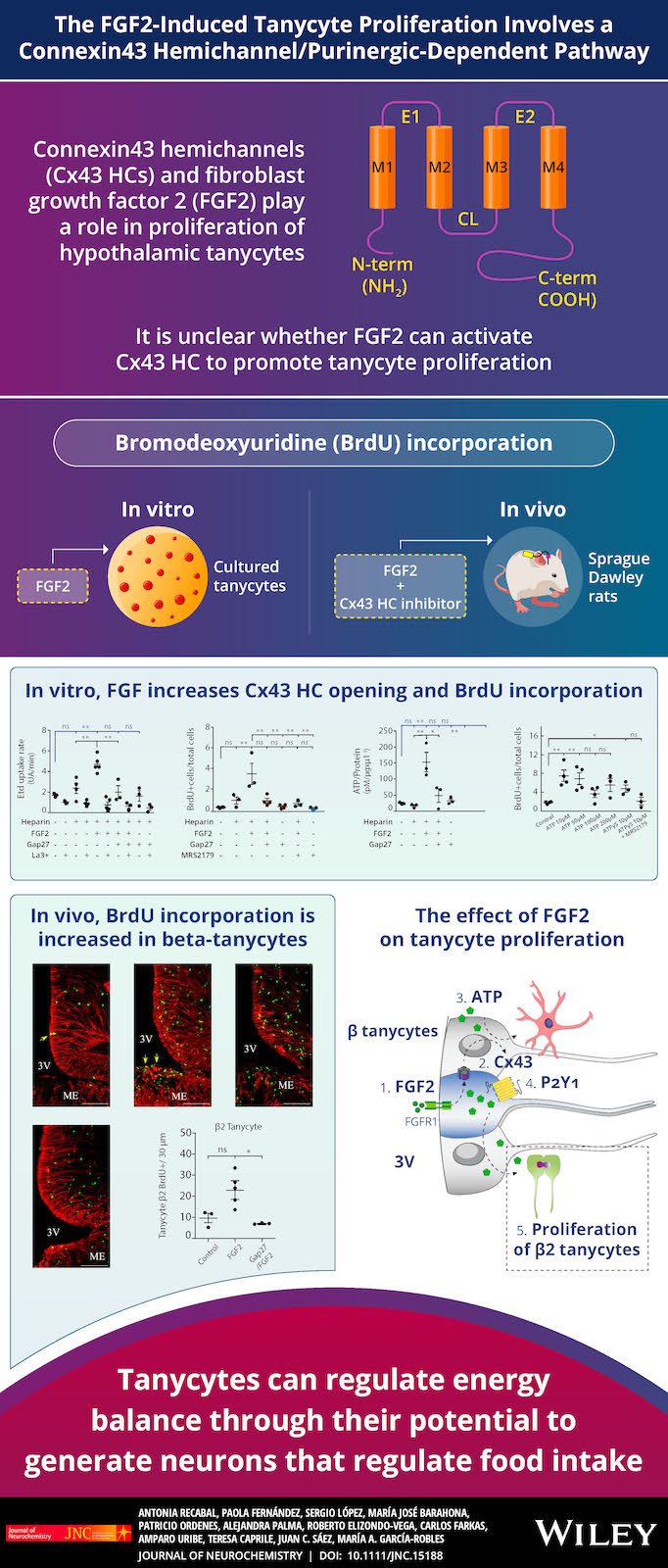
Our previous findings have led us to propose the existence of metabolic coupling between tanycytes and neuronal cells in response to high glucose, where lactate and very likely the ATP produced by tanycytes participate. Moreover, we and others have shown that tanycytes display high glycolytic activity and efficiently respond to glucose with increased cytoplasmatic Ca2+, dependent on purinergic receptors. We are studying the molecular mechanism for which, in response to nutritional signals, tanycytes proliferate and differentiate into other glial or neuronal cells. Our recent data show that connexin 43 and purinergic receptors are involved.
Roberto Elizondo-Vega

Mesenchymal stem cells, immunometabolism and immunosuppression
Elizondo Lab study the role of cellular metabolism on pathologies of an inflammatory nature, proposing its study and modulation as a new therapeutic tool for the treatment of pathologies of high impact in society such as obesity and rheumatoid arthritis among other
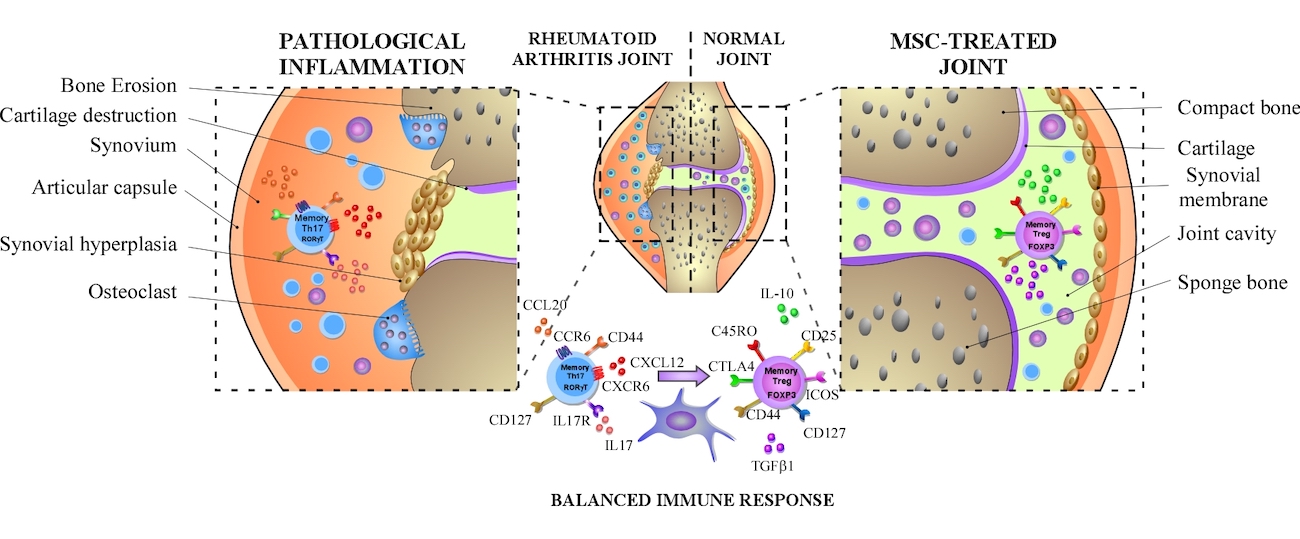
Chronic inflammation is a common denominator for a series of pathologies that are characterized by the presence of inflammatory cell infiltrates and a massive production of cytokines, producing a gradual loss of cellular homeostasis.
 Unfortunately, the mechanisms of chronic inflammation in a series of metabolic, autoimmune, neurodegenerative and cardiovascular pathologies are unknown.
Unfortunately, the mechanisms of chronic inflammation in a series of metabolic, autoimmune, neurodegenerative and cardiovascular pathologies are unknown.
Pathologies such as obesity or autoimmune diseases are characterized by high levels of pro-inflammatory cytokines and infiltration of inflammatory cells.
In the Immunometabolism and Stem Cell Laboratory, we are beginning to investigate how cell metabolism can modulate the immunosuppressive properties of mesenchymal stem cells (MSCs).
MSCs have excellent immuno-regulatory properties, due to their ability to inhibit the proliferation of pro-inflammatory cells, and induce the growth of cells with anti-inflammatory properties, establishing them as a therapeutic candidate for the treatment of inflammatory and auto-immune pathologies.
However, enthusiasm for the therapeutic use of MSCs has waned due to inconsistencies between the results obtained between pre-clinical models and clinical studies.
For this reason, there has been a growing interest in understanding how it is possible to enhance the therapeutic properties of MSCs, in which it has been suggested that the regulation of cellular metabolism and its knowledge, would be able to modulate the immunosuppressive properties of MSCs.
In vitro studies
We use transport techniques to assess incorporation and release of glucose or lactate, microscopy of cell live to evaluate calcium waves and pH changes in response to glucose and its metabolites in culture of hypothalamic glia and neurons.
In vivo studies
We have developed adenovirus that allows to inhibit or overexpress specifical proteins in the tanycytes through injection into the third ventricle. Lentivirus are commonly used to affect the expression of neuronal proteins. In these models we evaluate expression of neuropeptides that controls food intake and changes in the frequency of feeding.
In situ studies
We use brain and different tissues for immunolocalization assays using multiple labeling and spectral confocal microscopy.
Ex vivo studies
In sliced of hypothalamus we analyzing calcium waves and electrophysiological recording using transgenic mice (POMC-EGFP).
- Contreras-Lopez R*, Elizondo-Vega R*, Luque-Campos B, Torres MJ, Pradenas C, Tejedor G, Paredes-Martinez MJ, Vega-Letter AM, Campos-Mora M, Rigual-Gonzalez Y, Oyarce K, Salgado M, Jorgensen C, Khoury M, García-Robles MA, Altamirano C, Djouad F, and P. Luz-Crawford. 2021. The ATP synthase inhibition induces an AMPK dependent glycolytic switch of mesenchymal stem cells that enhances their immunotherapeutic potential. Theranostics 11 (1), 445-460 doi: 10.7150/thno.51631.
- Court AC, Le-Gatt A, Luz-Crawford P, Parra E, Aliaga-Tobar V, Bátiz LF, Contreras RA, Ortúzar MI, Kurte M, Elizondo-Vega R, Maracaja-Coutinho V, Pino-Lagos K, Figueroa FE, Khoury M. 2020. Mitochondrial transfer from MSCs to T cells induces Treg differentiation and restricts inflammatory response. EMBO Rep. 2020 Feb 5;21(2):e48052. doi: 10.15252/embr.201948052.
- Terraza-Aguirre C, Campos-Mora M, Elizondo-Vega R, Contreras-López RA, Luz-Crawford P, Jorgensen C, Djouad F. 2020. Mechanisms behind the Immunoregulatory Dialogue between Mesenchymal Stem Cells and Th17 Cells. Cells. 2020 Jul 10;9(7):1660. doi: 10.3390/cells9071660.
- Barahona MJ, Rojas J, Uribe EA, and García-Robles MA. 2020. Tympanic Membrane Rupture During Stereotaxic Surgery Disturbs the normal Feeding Behavior in Rats. Front. Behav. Neurosci.| https://doi.org/10.3389/fiber.2020.591204.
- Lobos M, Figueroa M, Martínez-Oyanedel J, López V, García-Robles MA, Tarifeño-Saldiviaa E, Carvajal N and Uribe E. 2020. Insights on the participation of Glu256 and Asp204 in the oligomeric structure and cooperative effects of human arginase type I. Journal of Structural Biology Volume 211, Issue 2, 1, 107533. doi.org/10.1016/j.jsb.2020.107533.
- Contreras-Lopez RA*, Elizondo-Vega R*, Paredes MJ, Luque-Campos N, Torres MJ, Tejedor G, Vega-Letter AM, Figueroa-Valdés A, Pradenas C, Oyarce K, Jorgensen C, Khoury M, Garcia-Robles MA, Altamirano C, Djouad F, Luz-Crawford P. 2020. HIF1α‐dependent metabolic reprogramming governs mesenchymal stem/stromal cell immunoregulatory functions. The FASEB Journal 34 (6), 8250-8264 doi: 10.1096/fj.201902232R
- Contreras-Lopez RA*, Elizondo-Vega R*, Torres MJ, Vega-Letter AM, Luque-Campos N, Paredes-Martinez MJ, Pradenas C, Tejedor G, Oyarce K, Salgado M, Jorgensen C, Khoury M, Kronke G, Garcia-Robles MA, Altamirano C, Luz-Crawford P& Djouad F. 2020. PPARβ/δ-dependent MSC metabolism determines their immunoregulatory properties. Scientific Reports 10 (1), 1-8 doi: 10.1038/s41598-020-68347-x.
- Recabal A, Fernández P, López S, Barahona MJ, Ordenes P, Palma A, Elizondo-Vega R, Farkas C, Uribe A, Caprile T, Sáez JC, García-Robles MA. 2020. The FGF2‐induced tanycyte proliferation involves a connexin 43 hemichannel/purinergic‐dependent pathway. J Neurochem. 156(2) doi: 10.1111/jnc.15188.
9. Reyes MB 1, Martínez-Oyanedel J, Navarrete C, Mardones E, Martínez I, Salas M, i López V, García-Robles MA, Tarifeño-Saldivia E, Figueroa F, García D and Uribe E. 2020. Insights into the Mn2+ Binding Site in the Agmatinase-Like Protein (ALP): A Critical Enzyme for the Regulation of Agmatine Levels in Mammals. Int. J. Mol. Sci. 21(11), 4132; https://doi.org/10.3390/ijms21114132
- Palma A, Konar M, Ordenes P, Maureira F, Elizondo-Vega R, Oyarce K, López S, Rojas J, Steinberg X. Garcia-Robles MA, Sepulveda F. 2019. Glucose increase DAGLa levels in tanycytes and its inhibition alters orexigenic and anorexigenic neuropeptides expression in response to glucose. Frontier Endocrinology 2019. Front Endocrinol 10:647. doi: 10.3389/fendo.2019.00647eCollection.
- Luque-Campos N, Contreras-López RA, Jose Paredes-Martínez M, Torres MJ, Bahraoui S, Wei M, Espinoza F, Djouad F, Elizondo-Vega RJ, Luz-Crawford P. 2019. Mesenchymal Stem Cells Improve Rheumatoid Arthritis Progression by Controlling Memory T Cell Response. Front Immunol. 2019 Apr 16;10:798. doi: 10.3389/fimmu.2019.00798.
- Uribe E, Reyes ME, Martínez I, Mella K, Salas M, Tarifeño-Saldivia E, López V, García-Robles M, Martínez-Oyanedel M, Figueroa M, Carvajal N, SchenkG. 2019. Functional analysis of the Mn2+ requirement in the catalysis of ureohydrolases arginase and agmatinase – a historical perspective. 10.1016/j.jinorgbio.2019.110812
- Elizondo-Vega RJ, Recabal A, Oyarce K. 2019. Nutrient Sensing by Hypothalamic Tanycytes. Front Endocrinol (Lausanne). 2019 Apr 16;10:244. doi: 10.3389/fendo.2019.00244. eCollection 2019.
- Elizondo-Vega R, Barahona MJ, Recabal A, Oyarce K, Ordenes P, Salgado M, Pincheira R, Luz-Cawford P, García-Robles MA. 2019. Inhibition of hypothalamic MCT4 and MCT1-MCT4 expression affect food intake and alter orexigenic and anorexigenic neuropeptide expression. Molecular Neurobiology 57 (2), 896-909. 2019. DOI: 10.1007/s12035-019-01776-6
- Salgado M, Ordenes P, Villagra M, Uribe E, García-Robles MA, Tarifeño-Saldivia E. 2019. When a Little Bit More Makes the Difference: Expression Levels of GKRP Determines the Subcellular Localization of GK in Tanycytes. Front Neurosci. 13:275. https://doi.org/10.3389/fnins.2019.00275
- Hermosilla V, Salgado G, Riffo E, Escobar D, Hepp MI, Farkas C, Galindo M, Morín V, García-Robles MA, Castro AF, Pincheira R. 2018. SALL2 represses cyclins D1 and E1 expression and restrains G1/S cell cycle transition and cancer-related phenotypes. Mol Oncol. 12(7):1026-1046. DOI: 10.1002/1878-0261.12308
- Recabal A, Elizondo-Vega R, Philippot C, Salgado M, López S, Palma A, Tarifeño-Saldivia E, Timmermann A, Seifert G, Caprile T, Steinhäuser C, García-Robles MA. 2018. Connexin-43 Gap Junctions Are Responsible for the Hypothalamic Tanycyte-Coupled Network. Front Cell Neurosci. 2018 12:406. DOI: 10.3389/fncel.2018.00406
- Kurte M, Luz-Crawford P, Vega-Letter AM, Contreras RA, Tejedor G, Elizondo-Vega R, Martinez-Viola L, Fernández-O’Ryan C, Figueroa FE, Jorgensen C, Djouad F, Carrión F. 2018. IL17/IL17RA as a Novel Signaling Axis Driving Mesenchymal Stem Cell Therapeutic Function in Experimental Autoimmune Encephalomyelitis. Front Immunol. 2018 Apr 30;9:802. doi: 10.3389/fimmu.2018.00802.
- Barahona MJ, Llanos P, Recabal A, Escobar-Acuña K, Elizondo-Vega R, Salgado M, Ordenes P, Uribe E, Sepúlveda FJ, Araneda RC, García-Robles MA. 2018. Glial hypothalamic inhibition of GLUT2 expression alters satiety, impacting eating behavior. Glia. 2018 doi: 10.1002/glia.23267
- Benítez J, García D, Romero N, González A, Martínez-Oyanedel J, Figueroa M, Salas M, López V, García-Robles M, Dodd PR, Schenk G, Carvajal N, Uribe E. Metabolic strategies for the degradation of the neuromodulator agmatine in mammals. Metabolism. 2018 Apr;81:35-44. doi: 10.1016/j.metabol.2017.11.005
- Uranga RM, Millán C, Barahona MJ, Recabal A, Salgado M, Martínez F, Ordenes P, Elizondo-Vega R, Sepúlveda F, Uribe E, García-Robles MA. 2017. Adenovirus-mediated suppression of hypothalamic glucokinase affects feeding behavior. Sci Rep. 1;7(1):3697. doi: 10.1038/s41598-017-03928-x
- Romero N, Benítez J, Garcia D, González A, Bennun L, García-Robles MA, López V, Wilson LA, Schenk G, Carvajal N, Uribe E. 2017. Mammalian agmatinases constitute unusual members in the family of Mn2+-dependent ureahydrolases. J Inorg Biochem. 122-125. doi: 10.1016/j.jinorgbio.
- Recabal A, Caprile T, García-Robles, MA. 2017. Hypothalamic Neurogenesis as an Adaptive Metabolic Mechanism. Front. Neurosci. 5;11:190. doi:10.3389/fnins. 2017.00190.eCollection
- Elizondo-Vega R, Cortés-Campos C, Barahona MJ, Carril C, Ordenes P, Salgado M1, Oyarce K, García-Robles MA. 2016. Inhibition of hypothalamic MCT1 expression increases food intake and alters orexigenic and anorexigenic neuropeptide expression. Scientific Reports. 28;6:33606
- Elizondo-Vega R, Salgado M, García-Robles MA. 2016. Monocarboxylate Transporters (MCTs) and their Role in Hypothalamic Glucosensing. MOJ Cell Sci Rep. 3(4): 00066
- García D, Ordenes P, Benítez J, González A, García-Robles MA, López V, Carvajal N, Uribe E. 2016. Cloning of two LIMCH1 isoforms: characterization of their distribution in rat brain and their agmatinase activity. Histochem Cell Biol. 145(3):305-13.
- Elizondo-Vega R, Cortes-Campos C, Barahona MJ, Carril C, Oyarce K, and García-Robles MA. 2015. The Role of Tanycytes in Hypothalamic Glucosensing. Journal of Cellular Molecular Medicine. J Cell Mol Med. 19(7):1471-82. DOI: 10.1111/jcmm.12590
- Quiñones M, Cofre J, Benítez J, García D, Romero N, González A, Carvajal N, García-Robles MA, López V, Schenk G, Uribe E. 2015. Insight on the interaction of an agmatinase-like protein with Mn(2+) activator ions. J Inorg Biochem. 145:65-9.
- Salgado M, Tarifeño-Saldivia E, Ordenes P, Millán C, Yañez MJ, Llanos P, Villagra M, Elizondo-Vega R, Martínez F, Nualart F, Uribe E, García-Robles MA. 2014. Dynamic localization of glucokinase and its regulatory protein in hypothalamic tanycytes. PLoS One. 9(4):e94035. doi: 10.1371/ journal.pone.0094035
- Salazar K, Cerda G, Martínez F, Sarmiento JM, González C, Rodríguez F, García-Robles M, Tapia JC, Cifuentes M, Nualart F. 2014. SVCT2 transporter expression is post-natally induced in cortical neurons and its function is regulated by its short isoform. J Neurochem. 130(5):693-706. doi: 10.1111/jnc.12793
- Cortes-Campos C, Elizondo R, Carril C, Martinez F, Boric K, Nualart F, Garcia-Robles MA. 2013. MCT2 expression and lactate influx in anorexigenic and orexigenic neurons of the arcuate nucleus (ISI) PLoS One. 26;8(4):e62532.doi: 10.1371/journal.pone.0062532.
- Montoya F, Martínez F, García-Robles M, Balmaceda-Aguilera C, Koch X, Rodríguez F, Silva-Álvarez C, Salazar K, Ulloa V, Nualart F. 2013. Clinical and experimental approaches to knee cartilage lesion repair and mesenchymal stem cell chondrocyte differentiation. Biol Res. 46(4):441-51. doi:10.4067/S0716-97602013000400015
- Ulloa V*, García-Robles M*, Martínez F, Salazar K, Reinicke K, Pérez F, Godoy DF, Godoy AS, Nualart F. 2013 Human Choroid Plexus Papilloma Cells Efficiently Transport Glucose and Vitamin C. J Neurochem. 127(3):403-14. doi:10.1111/jnc.12295
- Nualart F, Castro T, Low M, Henríquez JP, Oyarce K, Cisternas P, García A, Yáñez AJ, Bertinat R, Montecinos VP, García-Robles MA. 2013. Dynamic expression of the sodium-vitamin C co-transporters, SVCT1 and SVCT2, during perinatal kidney development. Histochem Cell Biol. 139(2):233-47
- Nualart F, Salazar K, Oyarce K, Cisternas P, Jara N, Silva-Álvarez C, Pastor P, Martínez F, García A, García-Robles MA, Tapia JC. 2013. Typical and atypical stem cells in the brain, vitamin C effect and neuropathology. Biol Res. 45(3):243-56
- García-Robles MA, Elizondo R, Cortés-Campos C, Martínez F, Nualart F. 2012. Brain monitoring of glucose homeostasis. Neuron-glia interactions. Biomedical Research 3(1):29-39
- Balmaceda-Aguilera C, Cortés-Campos C, Cifuentes M, Peruzzo B, Mack L, Tapia JC, Oyarce K, García MA, Nualart F. 2012. Glucose transporter 1 and monocarboxylate transporters 1, 2, and 4 localization within the glial cells of shark blood-brain-barriers. PLoS One. 7(2):e32409. doi: 10.1371/journal.pone.0032409.
- Orellana JA, Sáez PJ, Cortés-Campos C, Elizondo RJ, Shoji KF, Contreras-Duarte S, Figueroa V, Velarde V, Jiang JX, Nualart F, Sáez JC, García MA. (2012) Glucose increases intracellular free Ca(2+) in tanycytes via ATP released through connexin 43 hemichannels. Glia. 60(1):53-68. doi: 10.1002/glia.21246.
- Nuñez-Parra A, Cortes-Campos C, Bacigalupo J, García MA, Nualart F, Reyes JG. (, 2011). Expression and distribution of facilitative glucose (GLUTs) and monocarboxylate/H+ (MCTs) transporters in rat olfactory epithelia. Chem Senses. 36(9):771-80. DOI: 10.1093/chemse/bjr052.
- Cifuentes M*, García MA*, Arrabal PM, Martínez F, Yañez MJ, Jara N, Weil B, Domínguez D, Medina RA, Nualart F. (2011). Insulin regulates GLUT1-mediated glucose transport in MG-63 human osteosarcoma cells. J Cell Physiol. 226(6):1425-32 DOI: 10.1002/jcp.22668
- Cortés-Campos C, Elizondo R, Llanos P, Uranga RM, Nualart F, García MA. 2011. MCT expression and lactate influx/efflux in tanycytes involved in glia-neuron metabolic interaction. PLoS One. 6(1):e16411. doi: 10.1093/chemse/bjr052.
- Mella C, Martínez F, García MA, Nualart F, Castro V, Bustos P, Carvajal N, Uribe E. 2010. Expression and localization of an agmatinase-like protein in the rat brain. Histochem Cell Biol. 134(2):137-44. doi: 10.1007/s00418-010-0720-z
- Millán C, Martínez F, Cortés-Campos C, Lizama I, Yañez MJ, Llanos P, Reinicke K, Rodríguez F, Peruzzo B, Nualart F, García MA. 2010. Glial glucokinase expression in adult and post-natal development of the hypothalamic region. ASN Neuro. 2(3):e00035. doi: 10.1042/AN20090059
- Nualart F, García MA, Medina R, Owen G. 2009. Glucose transporters in sex steroid hormone related cancer. Current Vascular Pharmacology 7: 534-548
- Caprile T, Salazar C, Astuya A, Cisternas P, Montecinos H, Millán C, García MA, Nualart F. (2009). The Na+-dependent L-ascorbic acid transporter SVCT2 expressed in brain stem cells, neurons and neuroblastoma cells is inhibited by flavonoids. Journal of Neurochemistry 108: 563-577
- Godoy A, Salazar K, Figueroa C, Smith GJ, García MA, Nualart F. 2009. Nutricional channels in breast cancer. Journal and Cellular Molecular Medicine 12(6): 1-12
- Meneses AM, Medina RA, Kato S, Pinto M, Jaque MP, Lizama I, García MA, Nualart F, Owen GI. 2008. Regulation of GLUT3 and glucose uptake by the cAMP signaling pathway in the breast cancer cell line ZR-75. J Cell Physiol. 214(1):110-6.
- Castro T, Low M, Salazar K, Montecinos H, Cifuentes M, Yáñez AJ, Slebe JC, Figueroa CD, Reinicke K, García MA, Henríquez JP, Nualart F. 2008 Differential distribution of the Sodium-vitamin C cotransporter-1 along the proximal tubule of the mouse and human kidney. Kidney International 74(10):1278-86
- Castro MA, Pozo M, Cortés C, García MA, Concha II, Nualart F. 2007. Intracellular ascorbic acid inhibits transport of glucose by neurons, but not by astrocytes. J Neurochem. 102(3):773-82
- Godoy A, Ulloa V, Rodríguez F, Reinicke K, Yañez AJ, García MA, Medina RA, Carrasco M, Barberis S, Castro T, Martínez F, Koch X, Vera JC, Poblete MT, Figueroa CD, Peruzzo B, Pérez F, Nualart F.2006.Differential subcellular distributionof glucose transporters GLUT1-6 and GLUT9 in human cancer: ultrastructurallocalization of GLUT1 and GLUT5 in breast tumor tissues. J Cell Physiol 207(3):614-27
- Astuya A, Caprile T, Castro M, Salazar K, García M, Reinicke K, Rodríguez F, Vera JC, Millán C, Ulloa V, Low M, Martínez F, Nualart F. 2005. Vitamin C uptake and recycling among normal and tumor cells from the central nervous system. J. Neurosci Res 79(1-2):146-56
- García MA, Salazar K, Millán C, Rodríguez F, Montecinos H, Caprile T, Silva C, Cortes C, Reinicke K, Vera JC, Aguayo LG, Olate J, Molina B, Nualart F. 2005. Sodium vitamin C cotransporter SVCT2 is expressed in hypothalamic glial cells. Glia 50(1):32-47
- Yáñez AJ, Bertinat R, Spichiger C, Carcamo JG, García M, Concha II, Nualart F, Slebe JC. 2005. Novel expression of liver FBPase in Langerhans islets of human and rat pancreas. J Cell Physiol. 205(1):19-24
- Silva-Alvarez C, Carrasco M, Balmaceda-Aguilera C, Pastor P, García MA, Reinicke K, Aguayo L, Molina B, Cifuentes M, Medina R, Nualart F. Ependymal cell differentiation and GLUT1 expression is a synchronous process in the ventricular wall. Neurochem Res. 30(10):1227-36
- Medina RA, Meneses AM, Vera JC, Gúzman C, Nualart F, Rodríguez F, García M, Kato S, Espinoza N, Monsó C, Carvajal A, Pinto M, Owen GI. 2004. Differential regulation of glucose transporter expression by estrogen and progesterone in Ishikawa endometrial cancer cells. J Endocrinol. 182(3):467-78
- Verleysdonk S, Hirschner W, Wellard J, Rapp M, Garcia MA, Nualart F, Hamprecht B. 2004. Regulation by insulin and insulin-like growth factor of 2-deoxyglucose uptake in primary ependymal cell cultures. Neurochem Res. 29(1):127-34
- García M, Millán C, Balmaceda-Aguilera C, Castro T, Pastor P, Montecinos H, Reinicke K, Zúñiga F, Vera JC, Oñate SA, Nualart F. 2003. Hypothalamic ependymal-glial cells express the glucose transporter GLUT2, a protein involved in glucose sensing. J Neurochem. 86(3):709-24
- Medina RA, Meneses AM, Vera JC, Guzmán C, Nualart F, Astuya A, García MA, Kato S, Carvajal A, Pinto M, Owen GI. 2003. Estrogen and progesterone up-regulate glucose transporter expression in ZR-75-1 human breast cancer cells. Endocrinology. 144(10):4527-35
- Klattenhoff C, Montecino M, Soto X, Guzmán L, Romo X, García MA, Mellstrom B, Naranjo JR, Hinrichs MV, Olate J. 2003. Human brain synembryn interacts with Gsalpha and Gqalpha and is translocated to the plasma membrane in response to isoproterenol and carbachol. J Cell Physiol. 195(2):151-7
- García MA, Carrasco M, Godoy A, Reinicke K, Montecinos VP, Aguayo LG, Tapia JC, Vera JC, Nualart F. 2001. Elevated expression of glucose transporter-1 in hypothalamic ependymal cells not involved in the formation of the brain-cerebrospinal fluid barrier. J Cell Biochem. 80(4):491-503
61.García, MA, Weigert G, Duk S, Alarcón, M. 1999 Chromosome aberrations study in human lymphocytes from marijuana smokers. Bull. Environ. Contam. Toxicol. 62:117-12.
- Rudolph MI, Cabanillas A, Gómez P, García MA, Villan L. 1997. On the mechanism of action of ethodin in inducing myometrium contractions. Gen Pharmacol. 28(3):381-5
- Rudolph MI, García MA, Sepúlveda M, Brandan E, Reinicke K, Nicovani S, Villan L. 1997. Ethodin: pharmacological evidence of the interaction between smooth muscle and mast cells in the myometrium. J Pharmacol Exp Ther. 282(1):256-61
- Brandan E. Melo F. García MA. Contreras, M. 1996 Significantly reduced expression of the proteoglycan decorin in Alzheimer`s disease fibroblasts. Clinical Pathol: Molec Pathol. 49:351-356.
- Pérez R, García MA, Arias P, Gallardo M, Valenzuela S, Rudolph MI. 1997. Inhibitionof xylazine induced uterine contractility by clenbuterol and nifedipine. Res Vet Sci. 63(1):73-6.